Abstract
This essay will look at the ways in which the hominid fossil record is interpreted, and the effects that the existence of a sister lineage to our own lineage has on those interpretations, specifically the sister lineage that is Paranthropus. Discussion begins with a focus on the various proposed phylogenies of the Paranthropus clade, and its relationship with associated hominid taxa. This is accompanied by some of the general problems and issues associated with determining phylogenetic relationships from the fossil record. This is followed by a discussion on the likely modes of evolution and evolutionary change that have been occurring in hominid evolution throughout the Pliocene and Pleistocene. A short discussion then takes place on the relevance of ecological theories such as the competitive exclusion principle in discussions on hominid evolution. The final chapters discuss the various theoretical and philosophical implications of the existence of the Paranthropus clade on the general perceptions and interpretations of hominid evolution and its modes thereof.
Phylogeny
The phylogeny of the early hominids discovered in South and East Africa is uncertain. To use the term Paranthropus to describe the robust Australopithecines presupposes the monophyleticism of the robust Australopithecine species, as correct taxonomic nomenclature requires genera to be monophyletic (Strait, Grine et al.: 1997: 18, Kemp: 1999: 53). However throughout this paper we will use the term Paranthropus to refer to the robust Australopithecines for convenience, but this is not meant as a presupposition of the monophyleticism of the robust Australopithecines.
The group of extinct hominids named Paranthropus were extant in South and East Africa around the late Pliocene and early Pleistocene. They were a craniodentally robust genus or grade, probably contemporary with other hominid species. It is generally considered that the robust species went extinct around 1-1.5 million years ago, whilst another hominid species lead to the genus Homo, and ultimately to us, Homo sapiens.
From the fossil record of Paranthropus, there have been several species identified, P. bosei, P. aethiopicus, and P. robustus. These species vary in craniodental attributes and have been separated in time and space from each other, as per Figure 1.
The phylogeny of the Paranthropus lineage is not known for certain, and there have been many theories suggested as to the various Paranthropus’ species phylogenetic relationships with the various other contemporary and adjacent (in both or either time and space) species of Hominids (Skelton, McHenry and Drawhorn: 1993, Grine: 1993, Strait, Grine, and Moniz: 1997). Most of the proposed phylogenies have similarities in the key areas, such as Paranthropus being a clade of ultimate extinction. Figure 1 shows a fairly typical phyletic tree.
Figure 1 - Typical Phyletic Tree (Strait, Grine, and Moniz: 1997: 55)
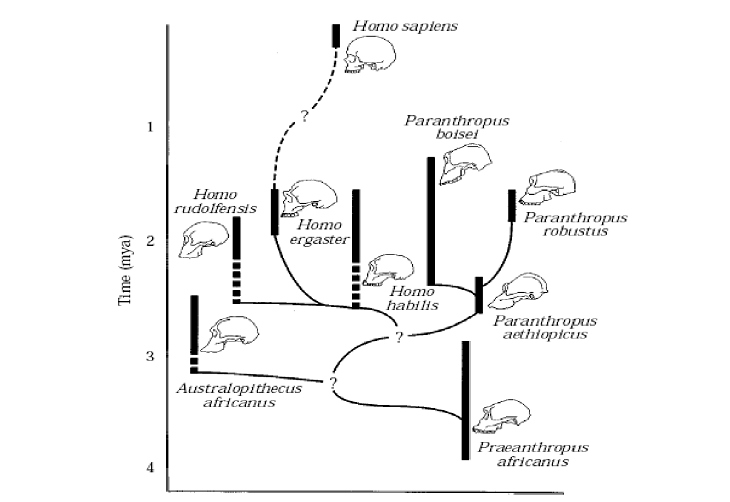
The recovered fossil record is naturally incomplete, and is in fact currently, and possibly always will be, inadequate for the purposes of accurately determining the true phylogeny of the Plio/Pleistocene hominids of South and East Africa, hence the multitude of proposals vying for acceptance as the true phylogeny (White: 1988: 449-451). This however does not stop some proposals being more likely to be true than others. There have been various attempts at quantifying the relevant hominid data and using computer software to analyse the data and suggest the most likely phylogenetic trees based on their relative parsimony values. However, recently the value of these techniques has been questioned, and some, (e.g. Hawks: 2004, and Collard and Wood: 2000), have shown the shortcomings of these techniques by using data on extant species where phylogenies are known through molecular studies.
Difficulties in determining the true, or at least an uncontroversial, phylogeny for the Paranthropus species is caused primarily by the difficulties in identifying palaeospecies, and the occurrences of homoplasy within the palaeospecies (Grine: 1988: 513 and McHenry: 1994: 6780). Species are difficult to identify in the fossil record due to the scarcity of the actual recovered record, and the general confusion as to what an actual species is, particularly when defined as the dynamic entities they are, varying through the dimensions of space and time. Homoplasy, convergent evolution, undoubtedly occurs in the evolution of species, and so causes difficulties in determining the evolutionary proximity of similarly adapted species such as Paranthropus robustus and Paranthropus boisei (Grine: 1988: 513).
Throughout the period of time for which research in to early hominids has been taking place, the general trend in proposed phylogenies has been from simple phylogenies with few species, to complex phylogenies with many species (Grine: 1988: 509, Tattersall: 2002: 1-2, Oakley: 1968: 258, Conroy: 1997: 252, Lewis and Towers: 1969: 93, Foley: 1987: 381). This progression could only really happen in the direction that it did, or not at all. In the beginning researchers had a much more limited range of fossil material with which to work, and so to create complex phylogenies with many species would have required the considerable use of one’s imagination and creativity. However with the large increases in the recovered fossil record, changes and adaptations have had to be made to previously proposed phylogenies. Newly found fossil remains have required the creation of new species, new genera, alterations in phylogenies, and alterations in accepted ranges of variation within species (Leakey, Tobias, and Napier: 1964). It is now thought that the phylogenetic tree of early hominids was considerably bushier than previously thought (Tattersall: 2002: 1, Foley: 1987: 381). A bushy tree means there must have been at least two species of hominids extant throughout the relevant time period (White: 1988: 454 and 464). However, this does not necessarily mean that two or more species were strictly sympatric (White: 1988: 454).
Further Phylogeny
Early interpretations of the hominid clade were of an anagenetically evolving unilinear lineage (Tattersall: 2002: 1, Conroy: 1997: 295, Foley: 1987: 381, Lewis: 1974: 74). This interpretation was made based on the very limited fossil record (Tattersall: 2002: 1), and on applications of ecological theories such as Gause’s (1934) competitive exclusion principle (Wolpoff: 1988: 485). It was deemed that culture represents a fundamental ecological niche, and therefore no two sympatric species can possess the trait that is culture (Tattersall: 2002: 1-2, Wolpoff: 1971: 601, Brace: 1967). With the limited fossil record available it was possible to arrange the fossils in a unilinear sequence. Indeed with the limited fossil record available it may have been unduly rash to begin arguing that the hominid sequence was not unilinear. The oft quoted principle of ‘Occam’s Razor’ could be appropriately used against such speculation, despite the fact that further research would show such speculation to be true.
However, with the furthering of the fossil record, more detailed studies of, and better understandings of, the fossils, some people began to question the putative simplicity of the hominid lineage (Leakey, Tobias, and Napier: 1964, Tattersall: 2002: 1, Foley: 1987: 381, Robinson: 1963: 601). There were attempts to reconcile various data with the putative lineage (Wolpoff: 1971: 611-2, Tattersall: 2002: 1), but eventually the data became overwhelming that multiple hominid species had existed sympatrically (Tattersall: 2002: 1, Oakley: 1968: 268-9, Robinson: 1963: 601, Clark: 1955: 8). Using the assumption that the fundamental hominid ecological adaptation was culture, and Gause’s competitive exclusion principle it was purported that only one of the then extant species could have been exercising culture as an adaptation (Wolpoff: 1961: 601, Brace: 1967). Obviously the species chosen as the exerciser of the culture, if there could only be one, would be the hominid presumed to be on our own direct lineage (Leakey, Tobias, and Napier: 1964: 9, Oakley: 1978: 269).
Mono- Para- and Poly- phyleticism
“genealogical relationships…have to be in the objectively testable form of proposed monophyly” (Kemp: 1999: 53)
A genus in reality should be monophyletic, just as a family, class, and order should be (Kemp: 1999: 53). It is easier for us to be certain of the monophyly of a grouping, the higher up the taxonomic rankings we go. However our ability to reconstruct the correct monophyletic phylogenies of extinct genera and species from the scant fossil remains we have is limited. Cela-Conde and Ayala (2003: 7685) citing Wood (1988) believe it is an “unrealistic taxonomic expectation” for genera to be monophyletic because “phylogeny is a continuum”. By this I believe Cela-Conde and Ayala (2003) could mean that it is hard to know where to place a species that lived close to the point of divergence of a species. This is because as species and individuals vary gradualistically it is difficult or impossible to tell which individual is ancestral to which other individuals and species circa to the locus of divergence, hence it is likely that genera will be polyphyletic or paraphyletic in reality, but we would not know for certain. Alternatively Cela-Conde and Ayala (2003) could mean that as “phylogeny is a continuum” speciation continues and hence new genera are necessarily created to encompass new species, therefore many palaeo-genera, and all palaeo-genera with currently extant ancestors, will not be monophyletic but paraphyletic.
Figure 2 – Theoretical Phylogeny – colours represent genera.
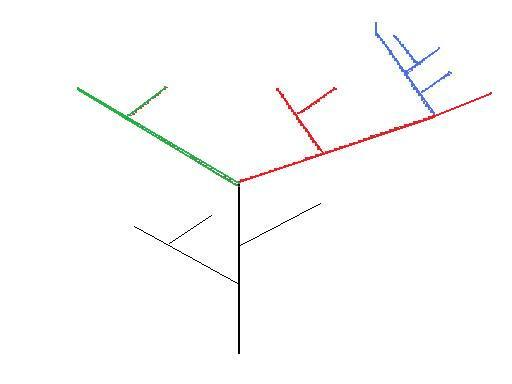
In this crude theoretical phylogenetic tree (Figure 2) the red lines could represent the genus Australopithecines, and the blue lines could represent Homo. In this case the Australopithecines are a paraphyletic group, but Homo is monophyletic. Whilst all the Australopithecines do share a common ancestry, (the black line), the taxon does exclude a lineage (Homo/blue) from the genus, and so is not monophyletic. Likewise, any of the individuals on the black lineage would be given a genus name which would also be paraphyletic. The clade containing the red and blue lineages, is however monophyletic.
One can say the Australopithecines are a monophyletic clade if all Australopithecines share a common ancestor. However it is not true that the genus Australopithecus is monophyletic if Homo evolved from an Australopithecine. In this case the genus of Australopithecus is paraphyletic, because it excludes some taxa that share the common ancestor of the Australopithecines (Strait, Grine, and Moniz: 1997: 17). If Australopithecus is a genus, and if an Australopithecine is the ancestor of Homo, then the Australopithecines are paraphyletic. What are the possible solutions? Homo could become a subgenus, or Australopithecus could become a tribe or supergenus. Alternatively Australopithecus and Homo could be combined to form one genus, with the associated various species and subspecies.
Figure 3 – Theoretical phylogenetic tree – colours represent genera
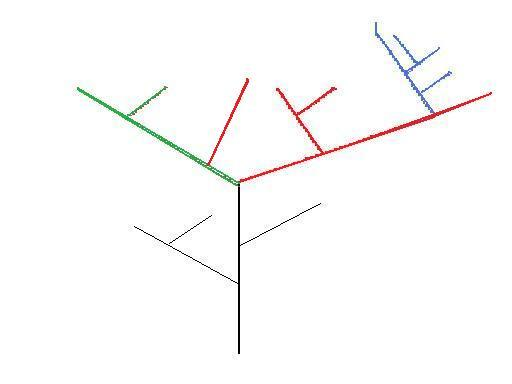
Figure 3 is an example of polyphyleticism. Polyphyleticisms should not be present in phylogenies at all, but occasionally they are suspected where previously a species has been presumed to belong to another lineage. Convergent evolution occurring in species which may have only diverged from a common ancestor a relatively short period of time prior (as in Figure 3), will make it difficult to figure out whether a specimen of either species belongs on the same lineage or not.
Current taxonomic nomenclature requires a specimen be given a genus name and a species name. This binomial is unique and should allow one to identify the species’ higher taxonomic rankings. At some time in the past, the ancestors of today’s species were discrete species themselves, and hence should be, and are, when known, assigned a binomial analogous to extant species. However this would then mean that should that ancestral species have given rise to further species and genera, then that genus itself will be paraphyletic. So perhaps it is nonsensical to suppose that palaeo-genera should be monophyletic. The genera obviously should not be polyphyletic, but could be either monophyletic or paraphyletic.
Figure 4 – A basic classificatory tree
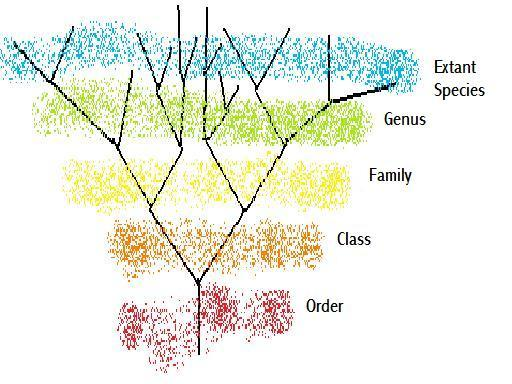
We do not have evidence of, and do not expect to ever have evidence of, the vast majority of species that ever existed. If we are studying only extant species, then a diagram such as Figure 4 suffices to show the relationships of species, as best we know, and each genus can be, and should be, monophyletic. However, when we start identifying fossil species, which may be located further back on the lineage than the extent of the current genus ranking, then we have to give them a genus and species name as per extant species. At one point in time there was a species that was the ancestor of all the families of a particular class. That species would not have as many higher taxonomic ranks as could be attributed to a currently extant species. Is this due to the finite beginnings of life on this planet, or to the poor resolution that we have from viewing these ancestral species from such a distant time? In response to this issue, proposals on varying taxonomic nomenclature have been put forward. It has been proposed that rather than give a species a specific binomial comprising of a species name and a genus name, a species should be described by its location on a phylogenetic tree. However if one were to maintain the regulation that the genus and species names (i.e. the last two names in the ‘phyletic location’ of the previous nomenclature) together form a unique identity then the prior parts of the species name/address would be unnecessary. This would leave you then with the same naming system except the binomial would not be specifically a genus and species name.
Australopithecus was originally given as a genus name to the group of species that formed something intermediate between apes and human. But this group of species was initially presumed to not be on the direct lineage leading to Homo, and so on its initial proposal it was a monophyletic genus. Likewise it was, with Paranthropus. However now it is proposed by many that an Australopithecine was ancestral to Homo, and proposed by a few that a Paranthropus was ancestral to Homo, thus making both genera potentially paraphyletic.
This problem has been tackled elsewhere by the addition of taxonomic rankings through time, as the complexity of phylogenies has become apparent, such as ‘family’, and ‘suborder’, as they have been needed to deal with certain lineages.
Species Evolution
When studying biological phylogenies, it is obviously important to understand how new species may arise. New species can arise through cladogenesis, or anagenesis. Anagenesis is the evolution of one species in to another species through time. Anagenesis occurs when a whole population of a species changes gradually through the generations. Cladogenesis is the evolution of one species in to two species, or one new species and the original species. Cladogenesis occurs when a species’ population diverges through generations in two different directions, or a part of a species population evolves differently from the original population which may remain in evolutionarily stasis. Cladogenesis is most likely to occur when a species’ population is split geographically.
Evolution is a gradual accumulation of mutations in a species over successive generations (Cela-Conde & Ayala: 2003: 7685). No individuals’ offspring will be noticeably different from the general population in any major way. Evolution occurs through the gradual shift of the mean of a variable trait, or a complex of variable traits, in a population through generations. This is micro evolution, or gradualistic evolution, but there is an alternative, termed saltational evolution. Saltational evolutionary change is when a large change occurs in one generation, for example, the addition of a limb (Kemp: 1999: 31). It is believed that saltational evolution only occurs in certain types of creatures, such as the insects with their segmented bodies. It is not believed that saltational evolution has occurred in any remotely recent evolutionary history of the hominids or their ancestors.
The pace of evolution is not known for certain, as it can rarely be seen in reality (Cela-Conde: 2003: 7685). Evolution can be observed through artificial selection of domesticates, both plants and animals, and through natural selection in species with a short generation time such as moths. The pace of early hominid evolution is debatable. It could be that species evolved relatively quickly in geological time and then remained in stasis for a longer period of geological time. Or it could be that hominid species were gradually evolving at a relatively steady, slow, rate throughout prehistory (Cela-Conde: 2003: 7685).
With the sparse fossil record we currently have it is not possible to determine whether early hominids evolved through punctuated gradualistic evolution, steady incremental evolution, or a combination of both to some extent, i.e. periods of relative fast evolutionary change followed be relatively slow evolutionary change (McHenry: 1994: 6785, Gould and Eldredge: 1993: 224). As we can not conduct a physical experiment to settle this, we have to rely on the advancement of theoretical judgement and the extrapolation of short term phenomena, and the advancement of the fossil record (Kemp: 1999: 21-22, Wood: 2000: 4).
Also, if one were to take McHenry’s (1994: 6785) view that most evolutionary change would be likely to occur on the fringes of a major species population, then our limited fossil record would of course be most likely to consist of the major population rather than the fringe population where evolutionary change would have been most likely to occur. This would mean that our fossil record may show a series of discrete species, but when there was nevertheless a gradual evolution between the species.
Gradual change does not necessarily equate to slow change. Gradual change is a series of small changes. Whilst this change may be slow on the scale of our lives, it will not necessarily appear slow in the fossil record.
With all the preceding in mind it becomes obvious that our taxonomic rules and nomenclature are ill equipped to deal with the definition of species through gradual time. Our taxonomic rules and nomenclature were originally developed with the presupposition that species were fixed entities in both time and space (Johanson and White: 1993: 107-8). However it is now apparent that species vary gradualistically through time and space. It is this discrepancy between our original ideals and reality that causes a lot of problems and fuels a lot of debates around species definitions.
Evolutionary pressures
Species evolve because of pressures put on them by their environment. Gould and Eldredge (1993: 344) suggest that the evolutionary pressures on early hominids must have been different to the previous pressures on the hominid/ape ancestors. They suppose this because had the evolutionary pressures been the same on early hominids and the hominid/ape ancestors, we would not have seen such a marked difference in traits (bipedality and encephalisation) that marked the beginning of the hominid radiation (Gould and Eldredge: 1993: 344). They suggest the rapid and large changes seen as the beginnings of the hominid radiation could only be caused by a change in the evolutionary pressures placed upon those species (Gould and Eldredge: 1993: 344).
Traits affect the chances of their bearers reproducing. Some traits have a stronger effect than others. Some traits, or trait complexes, can vary gradually, such as femur length, or height, whereas some other traits seem to exhibit more punctuated variation, such as eye colour, or whether or not one can roll their tongue. I believe that most traits can vary gradually, one could imagine pulling, pushing, stretching, and/or moulding a Homo sapiens, in to something very different. Evolution can do this to a species, but every infinitesimal stage along the way has to be evolutionarily ‘fit’ enough. Perhaps it doesn’t have to be the ‘fittest’, if it can still survive and pass on its genes then its offspring may be slightly ‘fitter’. The further from the ‘fittest’ an individual is, the less chance it has of passing on its genes. Therefore the most likely mode of change may be one that directly tracks a change in environment, so the species changes whilst remaining at its maximum possible ‘fitness’. Nevertheless a species can evolve through stages from one species to another through a complex of tiny changes. And in our hindsight it may appear that intermediary stages between two known species would have been relatively ‘unfit’. This may be an incorrect assumption based on a poor understanding of potential intermediary species, or it may be that in the current environment intermediary species as once existed would indeed be relatively ‘unfit’. However in the past environment the intermediary species may have in fact been relatively ‘fit’.
There are several species or sub species of baboons in Africa (Kamilar: 2006: 169-72). These groups of baboons are not entirely reproductively isolated from each other, and share almost identical ecological niches (Kamilar: 2006: 181). A group’s attributes remain stable and discrete except where interbreeding occurs where two groups meet. At these interbreeding localities are hybrid baboons exhibiting attributes intermediary between the two subject groups of baboons (Kamilar: 2006: 186-7). The hybrids are probably less ‘fit’ than the main populations. So these baboon groupings exhibit stasis over a wide geographical area and relatively sharp, but nevertheless gradual change, over a small area. However baboons also exhibit gradual change over the whole of their range in other traits, such as the trait complex controlling cranial shape and size (Kamilar: 2006: 189). So whilst some traits or trait complexes, exhibit stasis and punctuated change, other traits or trait complexes exhibit gradual change over geographic area, in what may be a single species (Kamilar: 2006: 189).
Such variety of change in extant geographical variation should be expected to occur in some extinct species as well. However it may also be that the variety of change described above may be to some extent analogous to the potential variety of change that occurs through time. Similar displays of clines of gradualism and punctuated equilibrium as exhibited by, for example, baboons, could be analogous to gradualistic clines and clines of punctuated equilibrium exhibited through temporal evolutionary variation. The differential rates of evolution exhibited by different parts of a body (McHenry: 1994: 6780) could be analogous to the different modes of clinal change exhibited by the baboons (Kamilar: 2006: 189).
Figure 5 – colours represent different times
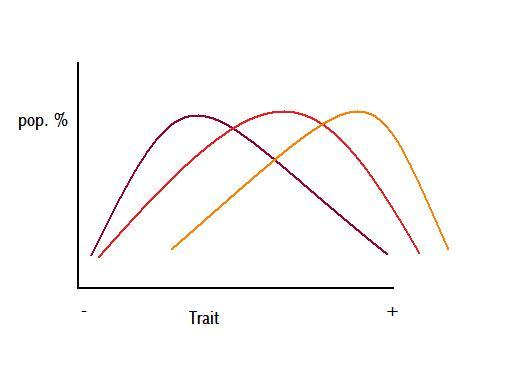
Figure 6
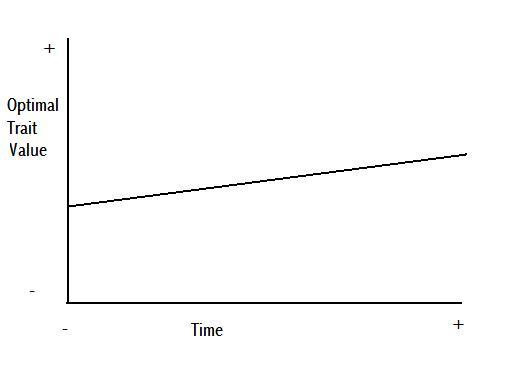
Figure 5 is a figurative demonstration of potential anagenetic change through time, where the three different colours, purple, red, and orange, represent a population at three consecutive intervals of time. This change could be caused by a gradual shift in the environment causing a gradual shift in the optimal value of a particular trait, as per Figure 6.
Figure 7
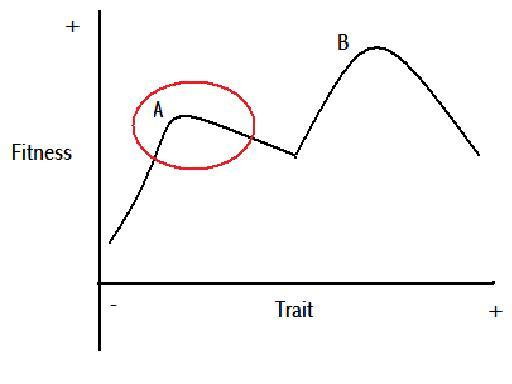
Figure 7 is a demonstration of the potential for two peaks in the fitness value of a scalar trait, after Nystrom et al.’s (2000) multi-equilibrium view of coral reef ecology. The part of the line within the red circle could represent the variation exhibited in a population. As the trait becomes more positive to the right of peak A, the traits fitness value reduces. That is, until a point roughly in the centre of the graph, where the fitness value begins increasing with a more positive trait. If peak A were to shift slowly in time to the right, as per Figure 5, the variation exhibited within the population should also shift to the right. When the population variation has shifted far enough to the right, some of the population at the extreme positive end of the trait will find themselves gaining an advantage from being at the extreme. This could lead to a major evolutionary event. If this event was coupled with the potential for reproductive isolation for the minority at the extreme positive end of the trait spectrum, cladogenesis could occur. If there was no reproductive isolation, the individuals with the extremely positive trait, could begin out competing those individuals with the less positive trait, and there would be a rapid anagenetic event. This is assuming the new found advantage does not remove the minority population from the ecological niche of the majority population. If the new found advantage of the extremely positive trait does suddenly open up a new ecological niche for the minority population, then cladogenesis could occur as the different ecological niches could create reproductive isolation, and the two populations would not be subject to the competitive exclusion principle.
The above possibility could be demonstrated imaginatively by any number of scenarios. One example could be this. A creature is 1 metre tall, just tall enough to reach the branches of a fruit tree, the fruit of which it eats. Being taller is no significant advantage, once it can reach the bottom branches it can climb throughout the tree to get the fruit. Nevertheless variation is exhibited in the species population, as variation is exhibited in many species. Some of the creatures are 1.10 metres tall, less of them are even taller at 1.20 metres tall. Some are 0.90 metres tall, but these creatures are at a significant disadvantage, there are not many trees around with the branches low enough for them to reach. So the optimum height for this creature is 1 metre, if it is smaller it can not reach the branches of the trees, if it is taller, it is a waste of good energy. However, there is another type of tree in this creature’s environment, a tree with a much more nutritious fruit, but this tree’s branches do not start until they are 1.5 metres above the ground. If in time the fruit tree on which the creature depends tends to increase the height of its first branches, subject to evolutionary pressures such as grazing of lower leaves by deer, then the subject creature’s optimum height will also increase in time. If the fruit tree’s lowest branches eventually come to begin only at the height of 1.3 metres, then the optimum height of our creature will also be 1.3 metres. Any shorter and it can not reach the branches to feed, and taller and it is a waste of energy. But still of course, the population exhibits variation. But now, if the tallest of the creatures is able to reach the smallest of the second type of fruit tree, that tall creature will suddenly acquire a massive advantage over his kin. Now the tallest of the population will reproduce more than the shorter ones, and, assuming a continuous interbreeding population, the average height of the population will increase rapidly to the new optimum of 1.5 metres, the height of the lower branches of the more nutritious fruit tree. As the mean height changes through time, more opportunities may arise such as the above described fruit tree. One change may lead to another and so on. There may be another type of tree slightly taller again, which is then attainable by those individuals at the taller end of the new average. The greater the change, the greater the chance that the change will result in further change, by opening up previously unavailable ecological niches for exploitation.
Figure 8 – anagenetic punctuated equilibrium
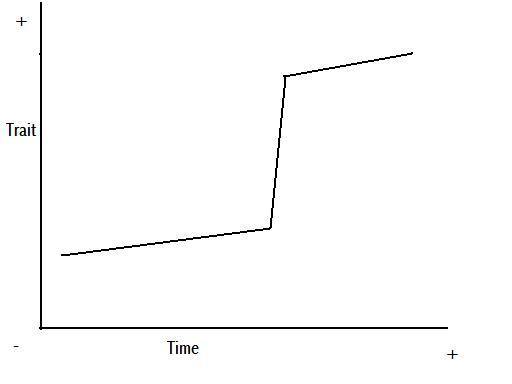
Figure 9 – cladogenetic punctuated equilibrium
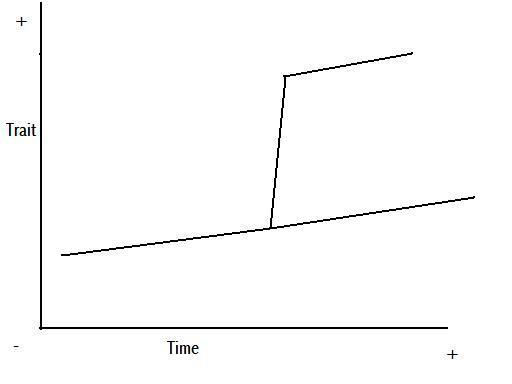
Figures 8 and 9 are representations of the potential change we might see in a trait through time, based on Figures 5 and 7, and the above explanation.
Analogous to this potential variation through time is Endler’s (1977) descriptions of spatial variation within and between species. These descriptions of species spatial variation could be seen as analogous to species variation of the gradual and punctuated equilibrium nature. It could also be that the geographic variation of species as described by Endler (1977) could be responsible for the patterns resultant from the recovered fossil record of Paranthropus and the rest of the Hominids.
Evolutionary pressures on Paranthropus
Paranthropus grade hominids evolved in a different way to non-Paranthropine hominids. Paranthropus grade hominids evolved hyper robust masticatory apparatus relative to the other contemporary hominids, and also relative to all hominids before and after the Paranthropines. It is therefore likely that the Paranthropine hominids had unique evolutionary pressures working on them, to cause this unique adaptation, just as it is likely that Homo grade hominids had unique evolutionary pressures working on them resulting in their distinctive hyper-encephalisation (Gould and Eldredge: 1993: 344). It has been suggested, logically, that the masticatory evolution seen in the Paranthropus grade hominids was an adaptation to eating tougher food stuffs (Wood and Strait: 2003: 119). These food stuffs have been proposed to be ground tubers, nuts, and other foods requiring high force mastication. However the Paranthropine masticatory adaptation, whilst initially interpreted as a specialisation (Wood and Strait: 2003: 119), has more recently been interpreted as a generalist evolutionary development (Wood and Strait: 2003: 154).
With the temporal and spatial resolution we currently have of hominid fossils circa the relevant time period, it is difficult to speculate on the circumstances of the evolution of Paranthropine grade hominids. Did they arise in a monophyletic manner, or did the Eastern (P. boisei) and Southern Africa (P. robustus) Paranthropus species evolve from separate populations and species of gracile Australopithecines?
Morphospace
The idea of representing species as definable entities in a hyperspace, where each dimension equates to a specific trait has been suggested numerous times (Kemp: 1999: 66, Shaklee and Shaklee: 1975). Whilst actually doing so for even one specimen would be an impossible task due to the massive number of variables, the idea can still be of use (Kemp: 1999). An individual can be seen to represent a point in morphological hyperspace (Kemp: 1999: 66). That point in morphological hyperspace equates to a hyper-volume in ecological hyperspace, representative of the niche in which that specimen can live (Shaklee and Shaklee: 1975: 611). The range of variation of traits from all individuals of a species at one particular time can be represented by a hyper-volume in morphological hyperspace. That hyper-volume in morphological hyperspace equates to a hyper-volume in ecological hyperspace. This gives the whole niche range of the species.
If there is in a species a potentially optimum collection of trait values, then it is possible in time the species population will tend towards that optimum. Then the longer period of time in which a species remains under the influences of one environment, the closer the average traits of the population will come to the optimum. The closer a population comes to an optimum suite of traits, the less variety will be exhibited in a population as a whole. If there is less variation in a population, a species will find it harder to evolve if environmental conditions change. If there is not enough variation in a species, the species will go extinct. If however in a species there is a range of trait variables, equally optimal when combined with other traits of varying values, then a species will not converge on one morphotype, but on a range of morphotypes. If there is this incipient variation in a species, then that species may be less likely to go extinct than a species which has only a single optimal morphotype, or even just a tighter range of optimal morphotypes, if or when the environment changes.
It appears to me that we are a very disparate species compared to most other species, domesticates aside. For example it seems to me that we are more disparate than chimpanzees. If this is the case, then through evolutionary time the hominid lineage has probably ultimately become more disparate. We now understand from the hominid fossil record that there have existed many hominid species, and many synchronic hominid species. Therefore there have been many hominid species extinctions. However the hominids have continued to survive, although now only as Homo sapiens, therefore hominid species have also done a lot of evolving in to other hominid species. Tracking the disparity of hominid species through their fossil record would be extremely difficult as it would require one to have a substantial record through both time and space. If we were to have fossils displaying the whole range of variation between the gracile Australopithecines and the Paranthropines, all jumbled up in time and space, then we could consider the possibility that the robust and the gracile Australopithecines were actually only one, albeit very disparate, species. This however, is unlikely to happen, as the furthering of the fossil record appears to be highlighting the discreteness of the gracile and robust grades, and even the discreteness of species within these grades. With the low temporal resolution possible in the dating of fossil hominid remains, even with a large fossil record of an individual species, it would be difficult to deduce whether the variety displayed by the group of fossils was evidence of disparity, or gradual change through time. The extent to which a species can vary can be useful in helping to determine palaeospecies from the fossil remains. Some species vary much more than others, but a common point of variation is sexual dimorphism.
Competitive exclusion principle - culture
The competitive exclusion principle dictates that no two sympatric species can occupy exactly the same ecological niche (Wolpoff: 1988: 485). This was used by some (Brace: 1967, Wolpoff: 1971, Wolpoff: 1988: 485) to argue that the hominid lineage must have been unilinear, because the fundamental hominid niche was culture (Brace: 1963: 90, Wolpoff: 1988: 485). They argued that “culture is a single ecological niche” (Brace: 1967), and that therefore “all hominid species occupy the same, extremely broad, adaptive niche” (Wolpoff: 1971: 601). However, by definition, no two species are identical. Each species varies in morphology, and in some cases culture. The cultural adaptation could not have sprung full blown in one generation of hominids, but must have been an adaptation occurring gradually over generations in a similar fashion to other adaptations (Wolpoff: 1988: 485). Therefore there must have been times when the cultural adaptation was different to what it is now, and different to what it has been at various times in the past (Wolpoff: 1988: 485). And so then there is no reason to suppose that the cultural adaptation could not have developed in different directions and at different rates in different species. It is only if one views culture as an all overriding adaptation that allows no variation in itself and discounts all other physical morphological adaptations of a specimen that one can reach the conclusion that no two species with the cultural adaptation can coexist. However suppose that two hominid species, both with some form of cultural adaptation, were to have different morphologies. Their cultural adaptation may allow them both to occupy the same ecological niches, but because their niches are so broad, each species can limit its niche and still survive (Wolpoff: 1988: 485-7). Their different morphologies may give them advantages in certain parts of their fundamental niches, thus allowing them to live sympatrically. Alternatively there differing morphologies may result in them having different fundamental niches, despite have a similar cultural adaptation.
Extinction
It is thought by most that the Paranthropus lineages went extinct around 1-1.5 mya, some however would disagree and argue for the persistence of Paranthropus through to historic times at least (Bayanov and Bourtsev: 1976: 316-7, Strasenburgh: 1975). The reasons for their extinction are, as with so many things, not known for certain, but several possibilities have been proposed. The most distinctive feature of the Paranthropines was there hyper robust crania, and particularly their mandibles and maxillae. It was thus proposed that it was this distinction which ultimately caused their extinction. Their hyper robust crania, conjunctive with sagittal crests were understood to be a development relating to high masticatory stress (Wood and Strait: 2003: 119-120). This adaptation was initially, and perhaps mistakenly, seen to be a specialisation, relative to the other then extant hominids such as the Australopithecines, and later early Homo (Wood and Strait: 2003: 119-120). It was proposed that Paranthropus had become over specialised with regards to diet, and when the climate changed, this reduced the availability of the food stuffs on which the Paranthropines had come to depend (Wood and Strait: 2003: 153). The Paranthropines were then unable to adapt in time to the changes in environment, due to their overspecialisation (Wood and Strait: 2003: 153). The Paranthropines therefore went extinct, and other, more generalised hominids, survived (Wood and Strait: 2003: 153). This view has been challenged by the argument that hyper robust crania are not a specialisation at all (Wood and Strait: 2003: 153-4). Whilst they are an adaptation, they do not necessarily limit the species in any way. It may be that the Paranthropines could eat everything the gracile Australopithecines could, and more. The hyper robust masticatory apparatus allowed the Paranthropines to eat tougher food, or food which required more and/or heavier mastication, but did not limit the other foods it could eat. Therefore the hyper robusticity exhibited by the Paranthropines made them more generalised species than would have been the case had they not developed their hyper robusticity. This would therefore invalidate the theory that the Paranthropines went extinct because of their dietary overspecialisation.
An alternative theory on the reason for the Paranthropine extinction is that they were out competed by early Homo. This argument can take two forms, in one it is proposed that Paranthropines and early Homo were sympatric and shared a similar ecological niche. This may have lead to competition between the Paranthropines and early Homo, which resulted in the extinction of the Paranthropines. In the other it is proposed that rather than out competing Paranthropines, early Homo affected a more direct extinctive effect on the Paranthropus grade hominids. This could take the form of ousting Paranthropines from their preferred areas, and/or directly hunting the Paranthropines. Evidence of Paranthropus and Homo overlapping in time and space for considerable periods could be evidence against linking Homo directly to the Paranthropine extinction.
Linked to the above theories of Paranthropine extinction is the proposal that the Paranthropines brain was restricted by the hyper-robusticity of the cranium. Masticatory muscles linking the jaw to the sagittal crest allow maximum musculature force to be applied to the masticatory system. However, it is proposed that this musculature, which effectively encompassed the skull, restricted encephalisation. With encephalisation restricted it is feasible that Paranthropus was out competed by a hominid which came to occupy a similar ecological niche, but ultimately had a bigger brain, and was therefore more intelligent and could possess a higher degree of culture. This bigger brain of the competing hominid genus Homo was then proposed to be the reason for the Paranthropine extinction. However, I do not see why the fact that the skull was encompassed in muscle related to the masticatory system would have necessarily restricted encephalisation. As the complex physiological beings that we and most other large organisms are, an adaptation often requires changes unrelated to the purpose of the adaptation itself. The masticatory muscles could probably still exert a substantial force on the jaws if the length to the sagittal crest was extended, and the braincase enlarged. Evolution does not work to increase an individual’s brain size, but selects over generations for those with a favoured trait. It may be that evolution of an adaptation occurs faster when the system is simpler, but I would not expect the extension of masticatory musculature apparatus to be a particularly complex system in extension, relative to some other adaptations.
Taphonomy
It has been shown from taphonomic studies that the most likely parts of hominin/ape skeletons to survive to fossilification are the craniodental portions (White: 1988, Wood and Strait: 2004: 123). This is amply reflected in the fossil record we have of early hominids. This preservational bias is due to the general robusticity of the craniodental skeletal material relative to the rest of the skeleton. This further limits our ability to determine and differentiate species as some species may not vary significantly in craniodental features. In fact there are extant examples of different species not even varying significantly through an entire skeleton (Wood and Richmond: 2000: 24). This demonstrates that we may not be able to differentiate between some extinct species where all that is preserved is the skeletal material, even if we have a comprehensive fossil record (Wood and Richmond: 2000: 24). There will therefore be a bias in palaeontology towards defining less species than in reality actually existed (Wood and Richmond: 2000: 24).
To one studying Hominid evolution, how important is the study of Paranthropus?
Discussion regarding the evolution of Homo sapiens need not be exclusively on that of Homo sapiens’ direct lineage. The discussion and understanding of a relatively recent side branch of Homo sapiens’ direct lineage may increase our understanding of the lineage of Homo sapiens (Grine: 1988: 509). This would be especially so, the closer the sister branch is to the direct line of Homo sapiens. The closest point one can get to between the direct Homo sapiens lineage and a sister branch, is obviously the actual branching point. This branching point is the first point in time that the Homo sapiens lineage takes a different evolutionary direction to the sister branch. It would undoubtedly be informative to study this branching point and determine the evolutionary pressures that caused a split in the lineage that led to two distinct genera, and several species. Many of these species in both genera were successful for long periods of time, but ultimately only the one species survived, that is, Homo sapiens.
From our perspective it is easy to see all species of hominids, except those on the direct line of Homo sapiens, as failures and evolutionary dead ends (Lewis and Towers: 1969: 80). Whilst ultimately this is true, it is not necessarily the most appropriate or enlightening way to view them. Many of these species survived for periods much longer than we as Homo sapiens have yet survived. These were not crippled creatures destined for disaster, but honed hominids existing for epochs.
Our species, Homo sapiens, is unique, it is special. This is a view I think many people would take, and justly so (Eiseley: 1954: 454, Bayanov and Bourtsev: 1976: 312). For on this planet we have no other rivals to our intelligence, our technology, our cultures, our language, or our manual dexterity. However this is a view developed through perception of only the three spatial dimensions, and maybe a relatively tiny distance through the fourth dimension, time. If however, we extend our perceptions to encompass the past several millions of years, we should come to realise we are not so unique, or so special (Eiseley: 1954: 454). Were we to trace back through time our own lineage, we would ultimately regress from our special attributes to those we might now attribute more generally to extant animal species. However the regression would be gradual. Each generation’s averaged attributes being imperceptibly different from the next, but nevertheless regressing over hundreds of thousands of years. If a sample of these generations, taken every couple of hundred thousand years or so, were currently extant, we would see species sharing attributes with us, in varying degrees, which we once considered unique and special, such as intelligence, manual dexterity, bipedalism, material culture, and language. However this visualisation is incomplete for the purposes of this essay. In addition to the generations of our own lineage, there are other lineages that branched off from our own lineage at various times in the past. These sister lineages may have also shared some attributes we now consider uniquely human. So we can now see that there were a whole host of other ‘species’ that had attributes we now consider uniquely human. There were both ‘species’ on our own direct lineage, and on sister lineages, displaying our human attributes to varying degrees, but also mixed with unique attributes of their own no doubt.
Once we have dismissed the undue notion of uniqueness and speciality commonly attributed to our species, we should be able to study the fossil hominid lineage more objectively. We can remove a bias that might have previously influenced our interpretation of the fossil record. This bias has been apparent in past interpretations of the hominid fossil record. Interpretations of fossil hominid sites and assemblages have at times been interpreted with assumptions, assumptions such as the exclusive nature of a cultural trait, or the unilinearity of the hominid lineage. At sites where stone tools have been found in conjunction with both Homo and Paranthropus species, previous interpretations have exclusively attributed the stone tools to the Homo species rather than the Paranthropus species. This prejudice was a result of the assumption that only a species on the direct lineage to Homo sapiens could have been responsible for the stone tools which represented a perceived fundamental and unique Homo sapiens attribute.
Biases
Ideally a science should be unbiased and objective. In the purer sciences such as physics and chemistry it is easier to be unbiased as the subjects of study are naturally more objective. Palaeoanthropology is more liable to biases, than physics for example, because of its more subjective nature as it is partially a social science, and partially a science based on limited data. Palaeoanthropologists construct their theories out of a very limited amount of data. It takes a long time for palaeoanthropologists to accumulate data, and so it is perhaps a consequence that palaeoanthropologists can then spend a long time working on a limited amount of data, and so may be liable to speculate subjectively, further than the data really allows. Speculation and subjectivity allows one to express biases. Palaeoanthropological data is often in fossil form. Fossils require interpretation, and that interpretation is often subjective. If one were just to stick to data which was formulated as objectively as possible, such as specific measurements and statements of fossils, one would have very little data to work with. Subjective interpretation is necessary in palaeoanthropology, but it is important to be aware of such subjectivity.
Biases expressed in academic literature are typically done so covertly. Either the author is not knowingly holding and expressing a biased opinion, or the author is knowingly expressing a bias covertly. It is acknowledged by everyone that science ideally should be free of bias, but that does not mean that nobody holds or expresses a bias, either knowingly or unknowingly. It is often the case that a whole scientific community holds a bias, in the form of a common sense assumption, and so the bias goes unchallenged (Foley: 1991: 53). In time when things change, with new scientists and new data, these biases may become glaringly obvious, and theories can be modified or discarded appropriately. We are well aware now of biases that have affected the field of palaeoanthropology and archaeology in the past (Foley: 1991: 53), but it is more difficult to know whether we currently hold any subconscious biases now. Palaeoanthropology is a relatively young science, compared with the more fundamental sciences, and so it has been more recently in time that we have become aware of biases in this field. I believe there are less biases, and less extreme bias, in current palaeoanthropology than in palaeoanthropology fifty or one hundred years ago, but only more time will allow us a more objective view on the relative biases of current palaeoanthropological work.
Before conclusive evidence was put forward that the hominid lineage was multilinear, it was supposed that the hominid lineage was unilinear (Robinson: 1963: 601), and had been distinct from the lineages of the great apes for circa 40 million years (Lewis and Towers: 1969: 93), on a continuous trend towards humanity (Tuttle: 1988: 392). The existence of Paranthropus contemporary with early Homo shows the non-unilinearity of the hominid clade (Oakley: 1968: 268-9). As various discoveries were made over time, theories were modified so as to fit with the new data. Theories were modified in such ways as to maintain underlying assumptions and biases about the human status.
“[I]mpatient people may prefer one of them [(Sivapithecus or Kenyapithecus)] to a blank page in our prehistory.” (Tuttle: 1988: 393). This implies people are more liable to extend the facts to pretend to understand our past more fully than we actually do. It is perhaps comforting to know our past. It is also comforting to continue believing what we believe we understand (Galbraith: 1958: 6). This is especially the case when what we believe we understand is acceptable socially, and to believe otherwise would involve conflict with self-interest and self-esteem (Galbraith: 1958: 6). As a partially social science, palaeoanthropology is liable to subjectivity as described by Galbraith (1958: 5-6). Galbraith (1958: 5) argues that “social phenomena…yield few hard tests”, therefore
“Within a considerable range he [or she]…may hold whatever view of this world he [or she] finds most agreeable" (Galbraith: 1958: 5).
Galbraith (1958: 6) further argues that whilst in the short term the truth may yield to the acceptable “conventional wisdom”, in the long term the “truth ultimately serves to create consensus”. Galbraith’s (1958) insights explain the development of palaeoanthropology through the decades very well, and allow us optimism in our current theories. Perhaps now we have broken away from the subjective “conventional wisdom” (Galbraith: 1958: 6) that prevailed in the earlier days of palaeoanthropology, and now perhaps we are closer to the truth of the hominid evolutionary past.
Wolpoff (1971: 611-12) argued that the gracile and robust Australopithecines were really only one, disparate, species, exhibiting variation analogous to variation seen across ethnic groups of our own, disparate, species. Wolpoff (1971) argued this case in order to make the data fit his suite of beliefs. He believed that only one species of hominid could exist at one time because ‘culture’ was a fundamental hominid adaptation, which would result in all hominids having the same broad ecological niche. Therefore using Gause’s competitive exclusion principle, two hominid species could not exist synchronically because as they had such a broad ecological niche, they would be extant over the whole of their potential range, and therefore be sympatric. If Wolpoff had been presented with data analogous to the data on the robust and gracile Australopithecines but in a context divorced from that of human evolution, he would possibly have interpreted the data differently, specifically, not attributed the gracile and the robust fossil material to a single species. If one believes one has an understanding of a process, one may be liable to attempt to fit new data in to that prior believed understanding, especially when that assumed understanding is of a subject of such a “personal nature” (Le Gros Clark: 1955: 4) as the study of our own past, (Galbraith: 1958: 5-6).
Conclusion
From the above we can see that there are few things that are certain in palaeoanthropology regarding the Paranthropus grade hominids. This is in part due to the short comings of the fossil record. Although through time we have learnt more, we have also learnt there is a lot more we have yet to learn. With fewer fossils with which to work, hominid evolution appeared simpler than it does today. Our evolutionary past is very complex, at one time we did not know how complex it was, at one time we did not even suspect how complex it was, and most probably even now we do not know how complex our evolutionary past was. However we do have a better idea about how complex our evolutionary past is now compared with how complex we thought it was one or two hundred years ago. This realisation of the complexity of our past has been brought about through the discoveries of an array of different hominid species, overlapping in time and space, including the Paranthropus grade hominids. The implications of these discoveries were that hominid evolution was not, simple, unilinear, and anagenetic, as once was assumed, but is complex, multilinear, bushy, and characterised by varied, or at least undetermined, modes of evolution. The further implications were that we are not as unique as historically we had believed, and in many cases presently still do believe. We are merely the end of a continuum of organisms that have been evolving for millions of years. Our eventual evolution was not predetermined or destined to be, but the result of a long series of environmental situations and events.
Are we still evolving along a gradual undetermined evolutionary continuum though? Many people would argue not. Many people would argue that we are no longer subject to natural selection as all species have been in the past, but have broken away from such natural bindings. Now we have developed such an advanced and complex culture can we determine our own evolutionary future? In welfare state societies, where the old, the ill, the feeble, the incapable, are looked after and survive where without help they would not, does this put a spanner in the works of natural selection? Perhaps evolution still occurs, but without natural selection as a guide to that evolution what would happen? Caring for the weak may put a spanner in the works of natural selection, but for how long have Homo sapiens, or their ancestors being doing so? I believe that such an altruistic practice of caring for the weak has long been practiced in human communities. It may be that the evolution of the welfare state is an extension of previous simpler systems in smaller scale societies, and that in turn is an extension of simple acts of reciprocal altruism as expressed by many organisms (Trivers: 1971: 35). The tendency to partake in acts of reciprocal altruism evolved as a useful and beneficial survival trait (Trivers: 1971: 35). I believe the complexity of the reciprocal altruism actions have increased through time in the hominid lineage.
The Paranthropus’ were one group of fossils determined by palaeoanthropologists to not be on the direct lineage of Homo sapiens, but also to have shared a common ancestor with the direct lineage of Homo sapiens, but to an ancestor which was not shared by any of the great apes. Therefore the Paranthropus’ were a hominid sister lineage to our own lineage. The existence and realisation of the Paranthropus grade hominids as being a sister lineage to our own helped modify our understanding of the evolutionary past of the hominids. It has helped extend broaden palaeoanthropologists minds with regard to variety of evolutionary situations that may have affected the various hominid lineages.
When studying the fossil record, we can see that it is important to be open to ideas and theories from all sources and fields. The fossil record is not a unique phenomenon, but the result of the preservation of life’s remains. Life has the same biological foundations and processes now as it did four million years ago, and so it is important to try and view the fossil record in light of the restrictions, processes, and evolutionary pressures that are extant today. The theory of evolution through natural selection has been well applied to the fossil hominid record. As have various ecological theories such as Gause’s (1934) competitive exclusion principle, which relate to the actual life processes of the species’ individuals. The patterns we find in the fossil record of the Paranthropus grade hominids can be variously explained by evolutionary and ecological theories. However with the variety of evolutionary and ecological models and theories extant, it is easy to find a model or theory to explain and support the fossil record. Likewise it is also easy to cite an ecological or evolutionary model or theory that does not fit with the facts of the fossil record. I believe that with the multitude of evolutionary and ecological theories that abound today one has to take with caution any proposal explaining the fossil record in these terms. Each evolutionary or ecological model may have a situation in which it works well or perfectly, but with the vast complexities of life and the environment I believe most theories will fail or need modification under most real life situations.
In the fossil record of Paranthropus can be seen various phenomena, such as some periods of what appear to rapid change, and some macroevolutionary gradual trends. But these types of evolutionary trends can be explained by combining various evolutionary theories. Likewise the potential presence of other hominids sympatric with Paranthropus can be explained by various ecological theories. However evolutionary and ecological theories have been used in the past to explain the phenomena we believed we observed in the fossil record. In time as what we came to believe was represented in the fossil record changed, so we also had to change the evolutionary and ecological theories used to explain the fossil record. This has happened specifically with various interpretations of the Paranthropus grade hominids. This leaves me cautious of accepting any of the various ecological and evolutionary theories as definite in explaining the hominid fossil record. Evolutionary and ecological theories may be useful in explaining the hominid fossil record, but they should not restrict our interpretation of the actual fossil evidence of our evolutionary past. Nevertheless a palaeoanthropological aim should still be to fit the hominid fossil record, and theories of ecology and evolution together.
It has been shown that biases have been expressed in academic literature on hominid evolution in the past. The discoveries of fossils required the reworkings of previous theories on hominid evolution. Theories and data were often manipulated in such ways to maintain assumptions developed before the discoveries of new fossils. Paranthropus fossil remains were one of the types of fossils discovered which clashed with prior theories. With time and further fossil discoveries, the interpretation of the Paranthropus fossils developed in to theories which fitted better with the available facts, and disregarded previous biases and assumptions.
Bibliography
Bayanov, D. and Bourtsev, I., (1976). On Neanderthal vs. Paranthropus, Current Anthropology, Vol. 17, pp. 312-318.
Brace, C. L., (1963). Article. American Journal of Physical Anthropology, pp. 87-91.
Brace, C. L., (1967). The Stages of Human Evolution, Prentice Hall, Engelwood Cliffs.
Cela-Conde, C. J., Ayala, F. J. (2003). Genera of the human lineage. Proceedings of the National Academy of Sciences, vol. 100, pp. 7684-7689
Collard, M., Wood, B.A. (2000). How reliable are human phylogenetic hypotheses? Proc. Natl. Acad. Sci. 97:
Conroy, G. C., (1997). Reconstructing Human Origins, W. W. Norton & Company Ltd., London.
Eiseley, L. C., The Reception of the First Missing Links. Proceedings of the American Philosophical Society, Vol. 98, No. 6, pp. 453-465.
Endler, J. A., (1977). Geographic Variation, Speciation, and Clines, Princeton University Press.
Foley, R. A., (1987). Hominid species and stone-tool assemblages: how are they related? Antiquity 61, pp. 380-392.
Foley, R. A., (1991). The Origins of Human Behaviour. Routledge.
Galbraith, J. K., (1958). The Affluent Society, Hamish Hamilton, London.
Gause, G. F., (1934). The Struggle for Existence, Williams & Wilkins, Baltimore, MD.
Gould, S. J. and Eldredge, N., (1993). Punctuated equilibrium comes of age. Nature, 366, no. 6452, pp. 223-227.
Grine, F. E., (1993). Australopithecine Taxonomy and Phylogeny: Historical Background and Recent Interpretation. in Ciochon, R. L., Fleagle, J. G. (eds.), The Human Evolution Source Book, Pp. 198-210. Prentice-Hall, Inc.
Grine, F. E., (1988). Evolutionary History of the “Robust” Australopithecines: A Summary and Historical Perspective. In Grine, F. E. (ed.), Evolutionary History of the “Robust” Australopithecines, pp. 509-520.
Hawks, J. (2004). How Much Can Cladistics Tell Us About Early Hominid Relations. American Journal of Physical Anthropology>i, 125: 207-219.
Howell, F. C., (1978). Hominidae. In Maglio, V. J. and Cooke, H. B. S. (Eds.), Evolution of African Mammals, Pp. 154-248.
Johanson, D. C., White, T. D., (1993). A Systematic Assessment of Early African Hominids. in Ciochon, R. L., Fleagle, J. G. (eds.), The Human Evolution Source Book, Pp. 100-111. Prentice-Hall, Inc.
Kamilar, J. M., (2006). Geographic Variation in Savanna Baboon (Papio) Ecology and its Taxonomic and Evolutionary Implications, in Lehman, S. M. and Fleagle, J. G., Developments in Primatology: Progress and Prospects, pp. 169-200.
Kemp, T. S., (1999). Fossils and Evolution, Oxford University Press.
Le Gros Clark, W. E. (1955). The Fossil Evidence for Human Evolution 1st edn, Univ. Chicago Press.
Leakey, L. S. B., Tobias, P. V., and Napier, J. R., (1964). A New Species of the Genus Homo from Olduvai Gorge. Nature, vol. 202, pp. 7-9.
Lewis, J., (1974). The Uniqueness of Man, Lawrence and Wishart, London.
Lewis, J. and Towers, B., (1969). Naked Ape or Homo Sapiens, Garnstone Press, London.
Lovejoy, C. O., (1981). The Origin of Man. Science, 23, Vol. 211, no. 4480, pp. 341-350.
McHenry, H. M., (1994). Tempo and mode in human evolution. Proc. Nat. Acad. Sci. USA, Vol. 91, pp. 6780-6786.
Oakley, K. P., (1968). The Earliest Tool-Makers. in Kurth, G., (ed.) Evolution und Hominisation, Gustav Fischer Verlag, Stuggart. Pp. 257-272.
Robinson, J. T., (1963). Australopithecines, Culture and Phylogeny, American Journal of Physical Anthropology, 21, pp. 595-605.
Shaklee, A. B. and Shaklee, R. B. (1975). Ecological Models in Relation to Early Hominid Adaptations, American Anthropologist, Vol. 77, pp. 611-615.
Skelton, R. R., McHenry, H. M., Drawhorn, G. M. (1993). Phylogenetic Analysis of Early Hominids. in R. L. Ciochon, J. G. Fleagle (eds.), The Human Evolution Source Book, Pp. 112-134. Prentice-Hall, Inc.
Strait, D. (2001). Integration, Phylogeny, and the Hominid Cranial Base, American Journal of Physical Anthropology, 114: 273-297.